An Aniline is a phenyl group linked to an amino group in an organic chemical molecule.
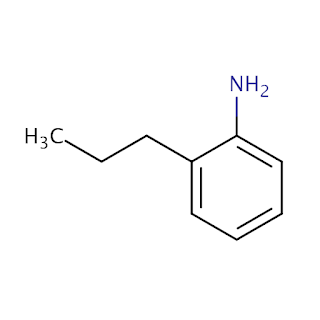 |
| Aniline |
Aniline is mostly utilised in the manufacturing of
polyurethane and other chemical goods. The market is expected to increase in
response to rising demand for aniline in rubber processing chemicals
applications. Rubber processing chemicals are employed in the processing of
latex because they increase the durability, robustness, strength, and elasticity
of the latex. Increased usage of additional chemicals utilised in the rubber
processing industries, such as antioxidants, stabilisers, antidegradants, and
antiozonants, is also predicted to boost market development.
Colours, medicines, explosives, plastics, and photography
and rubber compounds all employ aniline as an organic basis. The destructive distillation
of indigo yielded Aniline
for the first time in 1826. Its name comes from the indigo-producing plant
Indigofera anil (Indigofera suffruticosa), which has the chemical formula
C6H5NH2.
Commercial aniline is made via catalytic hydrogenation of
nitrobenzene or by ammonia action on chlorobenzene. The reduction of
nitrobenzene can also be done in aqueous acid using iron borings. An increase
in the usage of aniline raft in the coatings and textile sectors is expected to
drive market expansion. Furthermore, aniline is utilised in the pulp and paper
and pharmaceutical sectors, which is projected to boost the aniline market.
Furthermore, it is employed as a gasoline antiknock additive and solvent, which
is expected to drive the aniline market expansion throughout the forecast
period.
An Aniline
is a primary aromatic amine with a weak base that forms salts with mineral
acids. In an acidic solution, nitrous acid transforms aniline to a diazonium
salt, which is used as an intermediary in the production of a wide range of
colours and other commercially important organic compounds. When aniline reacts
with organic acids, it forms amides known as anilides, such as acetanilide,
which is made from aniline and acetic acid. Aniline and methyl alcohol can be
used to make monomethylaniline and dimethylaniline.
Cyclohexylamine is produced through catalytic reduction of
aniline. Aniline is converted to quinone, azobenzene, nitrosobenzene,
p-aminophenol, and the phenazine pigment aniline black by a variety of
oxidising agents. Pure aniline is a toxic, greasy, colourless chemical with a
nice odour.


Comments
Post a Comment