Amines; Compounds and Functional Groups That Contain a Basic Nitrogen Atom with a Lone Pair
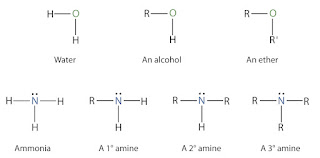
Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl and/or aryl group. Amines are classified as primary, secondary, or tertiary depending on whether one, two, or three of the hydrogen atoms have been replaced by organic groups. Functional groups of amine are found in a wide variety of compounds, including vitamins, polymers, natural and synthetic dyes, and medications such as codeine and penicillin. They are also found in many molecules essential to life, such as amino acids, neurotransmitters, hormones, and DNA. They are widely used in developing chemicals for crop protection, medication, and water purification.
Amines are organic compounds that contain a basic nitrogen atom with a
lone pair. They are formal derivatives of ammonia, with one of the hydrogen
atoms replaced with a substituent. Amines are derived from many sources,
including petroleum and food products. Amines are derived from ammonia, a
nitrogen-containing organic compound. They are naturally occurring organic
compounds, but they are also synthesized in laboratories. They are commonly
found in certain plants and are components of catecholamine neurotransmitters,
and are also involved in the production of amino acids, the building blocks of
proteins.
 |
| Amines |
They are similar to ammonia but have a
different smell. They are more complex than ammonia. They have a fishy,
decay-like aroma. The most common type of amides is trimethylamine, which is
often referred to as hawthorn blossom. It is also used in crop protection
chemicals and personal care products. It is important to note that ethanol
amines are the most common type of amide in the market. Amines are categorized
according to their structure and functionality. Generally, a 1o-amine is a
compound with only one alkyl group attached to the nitrogen atom. A 2o-amine
has two alkyl groups bonded together, whereas a 3o-amine has four alkyl groups.
An asymmetric molecule has a 4o-ammonium
cation on the nitrogen atom. Amines have high water solubility compared to
alcohols and ethers. This makes them highly soluble in water. However, a weaker
hydrogen bonding prevents them from being soluble in water. Despite this, there
are other applications of amines. They are commonly used in the production of
synthetic dyes, azo-dyes, and nylon. Amines are also used in personal care
products. Of all the types of amines, ethanol amides are the most common on the
world market. The most widely-used ones are ethanol amines and quaternary
ammonium salts.
Read
the complete Article- https://bit.ly/3sXEorv
The two latter compounds are more stable
than the former, but are characterized by a stereo genic center. The most
popular amines are methylamine and methamphetamine. Amines are not only used in
medicine but also in personal care products. Amines are the most common and
versatile organic compounds. They can be found in food, beverages, and
pharmaceuticals. Their unique properties make them a valuable commodity for
many industries. They are used in the production of food ingredients, textiles,
and clothing, and can be even used in a number of industries. They are extensively
used in the production of herbicide formulations, corrosion inhibitors, cement
and concrete, petroleum products, and chelating agents, among others. It is one
of the most widely used compounds in the world.


Comments
Post a Comment