The Global Hereditary Angioedema Market Was Valued At $1,563.7 Million In 2016 And Is Expected To Grow At A 9.1 Percent Cagr During The Forecast Period
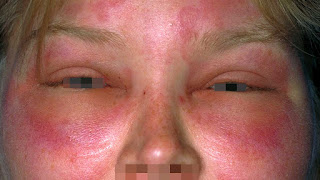
Hereditary angioedema (HAE) is a rare immune-related condition. The potentially fatal condition is caused by a lack of C1-esterase inhibitor (C1-INH), which causes blood vessels to dilate. The disease's symptoms include recurring episodes of edoema in various body parts such as the hands, feet, face, and airways. Based on the reduction in inhibitor synthesis or the formation of a dysfunctional protein, HAE is classified into three types: Type I HAE, Type II HAE, and Type III HAE. Although the condition is hereditary, the absence of a family history does not rule out the diagnosis of HAE, as up to 25% of HAE cases are caused by a spontaneous mutation of the C1-inhibitor gene at conception.
The global Hereditary Angioedema Market was valued at $1,563.7 million in 2016 and is expected to grow at a 9.1 percent CAGR during the forecast period (2017 - 2025). The growing number of hereditary angioedema cases, as well as the disease's hereditary nature, which allows the disease to pass the defective gene to future generations, are expected to drive market growth. According to a 2015 survey by the International Hereditary Angioedema Organization (HAEi), the global prevalence of hereditary angioedema is estimated to be 1 in 10,000 to 50,000 people, implying that approximately a quarter million people worldwide suffer from this rare and life-threatening deficiency condition.
Because of the greater number of drugs approved in the region, the presence of major manufacturers, and the presence of various organisations serving HAE patients, North America dominates the hereditary angioedema market. CSL Behring, Shire Plc., Pharming Healthcare, Inc., BioCryst Pharmaceuticals, Inc., Ionis Pharmaceuticals, Inc., Arrowhead Pharmaceuticals, Adverum Biotechnologies, and Attune Pharmaceuticals, Inc. are the major players in the global hereditary angioedema market. The FDA approved Haegarda (C1-esterase inhibitor) manufactured by CSL Behring for routine prophylaxis to prevent HAE attacks in adolescent and adult patients in 2017. However, the disease's rarity makes efficient diagnosis difficult, and patients must rely on alternative therapies to control the disease, which is one of the major factors limiting market growth.



Comments
Post a Comment